Submission Guideline
Submission Preparation Checklist
As part of the submission process, authors are required to check off their submission's compliance with all of the following items, and submissions may be returned to authors that do not adhere to these guidelines.
The submission has not been previously published, nor is it before another journal for consideration.
The submission file is in OpenOffice, or Microsoft Word document file format.
The text of the manuscript is double-spaced; uses a 12-point font; Time New Roman font, all illustrations, figures, and tables are placed within the manuscript.
Provide a website link to all references for PubMed, PMCID, DOI, Full Text (provide all or as much as available).
The ORCID IDs of all authors are mandatory.
The text adheres to the stylistic and bibliographic requirements outlined in the Author Instructions below.
For Research Article: Authors must check the EQUATOR Network, CONSORT and STROBE sites for any reporting guidelines that apply to your study design and ensure they include any required supporting information recommended by the relevant guidelines. Documentation (checklist) for specific studies should be uploaded as supporting information during manuscript submission.
For Case Report: Please download Case Report Consent Form, get it signed, keep the original with the patient chart and submit a copy of it.
Please add all co-authors in the list of contributors (with specific contribution).
Minimum word count required for submission: original (at least 15 references) and review article (at least 30 references) must be at least 2000 words and case report (at least 5 references) at least 1000 words.
Author Instruction
BJMR is a peer-reviewed, open-access journal that publishes multidisciplinary original articles, brief articles, case reports, review articles, research letters, letter to editors, perspectives, editorials and guest editorials. For detailed reporting guidelines for each type of article are described below. BJMR follows the International Committee of Medical Journal Editors’ recommendations for the conduct, reporting, editing and publication of scholarly work in medical journals. The uniform requirements and specific requirement of BJMR are mentioned below. Before sending a manuscript authors are requested to check for the latest instructions available.
Publication schedule
BJMR publishes one volume each year. Each volume consists of three issues which are published every four months months. The issues are published in January, May, and September of every calendar year.
Publication and decision time
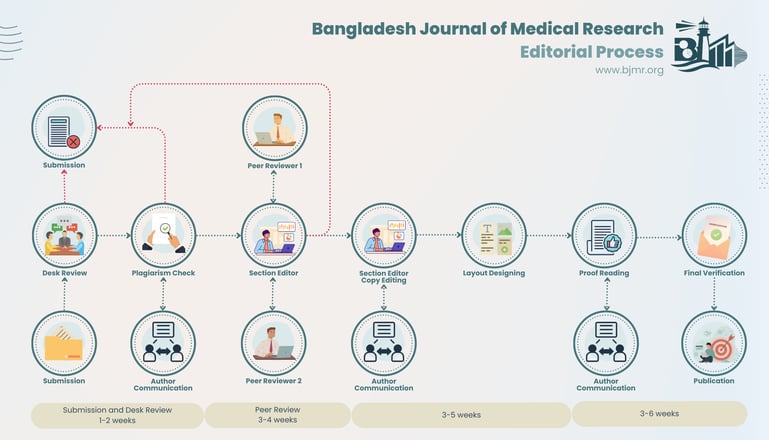
Time to first communication via email overall (average) within 2 weeks (with Desk Review); 4 weeks (with the review). Those articles which have been submitted more than six moths ago undergo auto-pruning (automatic declining). The editorial process is as follows:
If you find encounter delay and find no update on your BJMR submission, which could be due to;
i) submission was incomplete or
ii) submission was without following JNMA format and supplementary documents or
iii) there is an issue with your submission (e.g. ethical issues or research misconduct or related)
Because of any of the above issues or any other reasons, if you do not receive any information or update about your submission after 4 weeks, please contact JNMA as soon as possible. We will make sure that your voice is heard and addressed appropriately.
N.B. Please do not contact on personal email, social media or phone number to Editor-in-Chief, Editorial team or JNMA staff related to your submission. All communication must be made via JNMA official email address only. JNMA best editorial practice ensures that it publishes the highest standard articles.
Submission of manuscripts
All manuscripts need to be submitted online through https://www.banglajol.info/index.php/BSMMUJ/login along with the completed submission checklist and EQUATOR checklist available for different type of studies found at https://www.equator-network.org/. Manuscripts submitted that do not adhere to the prescribed 'Instructions to Authors' will be sent back to the authors for technical corrections before undergoing editorial or peer-review processes.
Manuscript submission requirements
Each submission must have the following docoments:
Authorship
Declaration
Manuscript
EQUATOR checklist (find relevant one from https://www.equator-network.org/reporting-guidelines/ )
Any submission without the above documents and manuscript not in BJMR format will be rejected outright. Therefore to avoid such errors and rejection, please submit your article with all supplementary and required files along with the use of appropriate template given below.
Types of manuscript with word limits
Original article:
Randomised controlled trials, interventional studies, studies of screening and diagnostic test, outcome studies, cost effectiveness analyses, casecontrol series and surveys with high response rate. Up to 3000 words excluding references (up to 30) and abstract (up to 250 words).
Review article:
Systemic critical assessments of literature and data sources. Up to 3000 words excluding references (>50 and <100) and abstract (250 words).
Case report:
New/interesting/very rare cases with clinical significance or implications can be reported. Up to 1000 words excluding references (up to 10) and abstract (up to 100 words), up to three photographs.
Viewpoint:
These articles are personal views and allow you to express your own point of view on any issues relevant to health. We like these to include controversial subjects. Up to 800 words excluding reference (up to 5- 8).
Letter to the editor:
Should be short, decisive observation. They should not be preliminary observations that need a later paper for validation. Up to 400 words and 5 references.
Manuscription preparation
All manuscripts should adhere to the JNMA format. The manuscript must be typed double-spaced on one side of an A4 size white paper with Arial Font (size 12). A minimum of 25 mm margins should be present. The pages should be numbered consecutively beginning from the title page. Numbers should be written at the top right.
To minimise the rejection (or return for revision) of your article please do the followings:
All the documents have to be submitted at once in single submission (as listed above).
Please use the appropriate template for your manuscript to avoid error in the heading and subheadings.
Please address all the point described in the template, EQUATOR checklist, references and manuscript preparation guidelines etc.
Please do not contact the editorial member's personal telephone numbers but BJMR office. If you have more queries, please contact us anytime. Help us to help you by providing the required information as described on this page.
Manuscript templates
Original article
Review article
Case report
Viewpoint
Letter to the editor
Original article
The eligible study types encompass randomized controlled trials, intervention studies, evaluations of screening and diagnostic tests, outcome studies, cost-effectiveness analyses, case-control series, and surveys with high response rates. The text of original articles amounting up to 3000 words (excluding abstract, references and tables) should be divided into sections with the headings Abstract, Keywords, Introduction, Methods, Results, Discussion, References, Tables and Figures. Original articles can have up to six tables or figures.
Abstract:
Abstracts should be within 250 words having background, methods, results, and conclusion sub-headings along with a maximum of five keywords.
Highlights:
This section should be no more than five bullet points relating to the strengths and limitations of this study specifically to the methods, not the results of the study. This will be published as a summary box after the abstract in the final published article.
Introduction:
Provide a context or background for the study (that is, the nature of the problem and its significance). State the specific purpose or research objective of, or hypothesis tested by, the study or observation; the research objective is often more sharply focused when stated as a question. Both the main and secondary objectives should be clear, and any prespecified subgroup analyses should be described. Provide only directly pertinent references, and do not include data or conclusions from the work being reported.
Methods:
The Methods section should contain study design, duration and place of study, ethical approval, patient consent (inclusion and exclusion criteria), sampling, statistical analysis and software used.
Study design: Clearly outline the process by which observational or experimental participants were chosen, whether they were patients, laboratory animals, or controls. Include detailed information about eligibility and exclusion criteria, as well as a description of the source population.
Selection and description of the participants: Describe your selection of the observational or experimental participants (patients or laboratory animals, including controls) clearly, including eligibility and exclusion criteria and a description of the source population. Because the relevance of such variables as age and sex to the object of research is not always clear, authors should explain their use when they are included in a study report; for example, authors should explain why only subjects of certain ages were included or why women were excluded. The guiding principle should be clear about how and why a study was done in a particular way. When authors use variables such as race or ethnicity, they should define how they measured the variables and justify their relevance.
Technical information: Identify the methods, apparatus (give the manufacturer's name and address in parentheses), and procedures in sufficient detail to allow other workers to reproduce the results. Give references to established methods, including statistical methods (see below); provide references and brief descriptions for methods that have been published but are not well known; describe new or substantially modified methods, give reasons for using them, and evaluate their limitations. Identify precisely all drugs and chemicals used, including generic name(s), dose(s), and route(s) of administration.
Reports of randomized clinical trials should present information on all major study elements, including the protocol, assignment of interventions (methods of randomization, concealment of allocation to treatment groups), and the method of masking (blinding), based on the CONSORT Statement (http://www.consort-statement.org). The reporting guidelines for other type of studies can be found at https://www.equator-network.org/reporting- guidelines/. The authors need to complete this checklist and send it with the submitted article.
Ethics: When reporting studies on human, indicate whether the procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional or regional) and with the Helsinki Declaration of 1975, as revised in 2000 (available at http://www.wma.net/e/policy/17-c_e.html). Do not use patients' names, initials, or hospital numbers, especially in illustrative material. When reporting experiments on animals, indicate whether the institution's or a national research council's guide for, or any national law on the care and use of laboratory animals was followed.
Evidence for approval by a local Ethics Committee (for both human as well as animal studies) must be supplied by the authors on demand. Animal experimental procedures should be as humane as possible and the details of anesthetics and analgesics used should be clearly stated. The ethical standards of experiments must be in accordance with the guidelines provided by the CPCSEA (animal) and ICMR (human). The journal will not consider any paper which is ethically unacceptable. A statement on ethics committee permission and ethical practices must be included in all research articles under the "Materials and Methods" section.
Statistics: In presenting research findings, authors are encouraged to quantify the results whenever possible and include appropriate indicators of measurement error or uncertainty, such as confidence intervals. Any losses to observation, such as dropouts in a clinical trial, should be reported. When summarizing data in the Results section, authors should specify the statistical methods employed for analysis. It is advisable to avoid using technical terms in a non-technical manner, such as 'random' (which implies a randomizing device), 'normal', 'significant', 'correlations', and 'sample'. It is essential to define statistical terms, abbreviations, and most symbols for clarity. Additionally, authors should explicitly state the computer software used in their analysis.
When reporting P values, use upper italics (e.g., P = 0.048), and provide the exact value rather than rounding it to 0.05 or 0.001. Mean differences for continuous variables, proportions for categorical variables, and relative risks, including odds ratios and hazard ratios, should be accompanied by their respective confidence intervals.
Results:
Arrange your findings in a logical order within the text, tables, and illustrations, placing the primary or most significant results first. Avoid duplicating all data presented in tables or illustrations within the text; instead, highlight or summarize essential observations. Additional or supplementary materials and technical details can be included in an appendix, allowing accessibility without disrupting the flow of the main text. Alternatively, such materials may be published solely in the electronic version of the journal.
When summarizing data in the Results section, provide numeric results not only as derived values (e.g., percentages) but also as the absolute numbers from which the derived values were calculated. Clearly specify the statistical methods employed for analysis. Limit the number of tables and figures to those necessary for elucidating the paper's argument and supporting its claims. In cases where tables have numerous entries, consider using graphs as an alternative but avoid duplicating data between graphs and tables. Furthermore, include analyses of the data based on relevant variables, such as age and sex, as appropriate from a scientific perspective.
Use graphs as an alternative to tables with many entries; do not duplicate data in graphs and tables. Avoid nontechnical uses of technical terms in statistics, such as 'random' (which implies a randomizing device), 'normal', 'significant', 'correlations', and 'sample'. Where scientifically appropriate, analyses of the data by such variables as age and sex should be included.
Discussion:
The discussion section should encompass a summary of the key findings, encompassing primary and secondary outcome measures, and their relation to prior hypotheses. It should also address the study's strengths and limitations, including aspects of the study question, design, data collection, analysis, and interpretation. Furthermore, the discussion should place the study's interpretation and implications within the broader context of existing evidence. This may involve referencing relevant systematic reviews or, if not available, considering the feasibility of conducting one. Authors should also highlight the study's contributions to the available evidence, its impact on patient care and health policy, and potential underlying mechanisms. Any controversies arising from the study's findings should be acknowledged. Additionally, the discussion should outline future research directions, including areas of focus for this particular research collaboration and potential avenues for clinical investigation.
Avoid repeating detailed data or material already presented in the Introduction or Results sections. In particular, refrain from making claims regarding economic benefits and costs unless economic data and analyses are included in the manuscript. Authors should not assert priority over unpublished work or make allusions to incomplete research. If necessary, new hypotheses may be introduced, but they should be clearly identified as such.
Conclusions:
Link the conclusions with the goals of the study but avoid unqualified statements and conclusions not adequately supported by the data. In particular, avoid making statements on economic benefits and costs unless the manuscript includes the appropriate economic data and analyses. Avoid claiming priority or alluding to work that has not been completed. State new hypotheses when warranted, but label them clearly as such.
Case reports
Unique, intriguing, and infrequent cases are eligible for reporting. Such cases should present exceptional diagnostic or therapeutic challenges, offering valuable learning opportunities for the readers. Priority will be given to cases with notable clinical significance or implications. These communications could be of up to 1000 words (excluding abstract and references) and abstracts within 150 words. Case reports should have the following headings: Abstract, Keywords, Introduction, Case description, Case management, Discussion, Reference, and Tables order. Up to three learning points of the report needs to be given in bullet points. The manuscript could be supported with up to 10 references. Case Reports could be authored by up to five authors.
Review article
Review articles are anticipated to be authored by individuals who possess extensive expertise and have conducted substantial research on the subject matter. The manuscript should be accompanied by a brief summary of the contributor(s)' work in the specific area of review.
The prescribed word count is up to 5000 words excluding tables, references and abstract. The manuscript may have any number of references as required. However, we prefer limiting the article within 80 references. The manuscript should have an unstructured abstract (up to 300 words) representing an accurate summary of the article. Up to six highlights of the article needs to be given in bullet points. The section titles would depend upon the topic reviewed. Authors submitting review article should include a section describing the methods used for locating, selecting, extracting, and synthesizing data. These methods should also be summarized in the abstract.
The journal requires the contributors to provide post-publication updates on the reviewed subject. These updates should be concise and highlight any significant advancements in the field that have occurred after the publication of the article. Contributors should submit these updates as letters to the editor whenever major developments arise.
The journal prefers systematic reviews that have been registered in PROSPERO https://www.crd.york.ac.uk/prospero/. The PROSPERO registry number should be provided in the review article under the "methodology" section.
Research letter
Research letters are peer-reviewed concise and focused scientific articles that communicate the key findings of a research study. They are shorter in length compared to full research papers and provide a succinct overview of the research process, results, and implications. These communications could be of up to 600 words. Abstracts within 80 words without any structure needs to be submitted. Abstracts will not be published but are required for DOI purposes. Up to two highlights of the letter needs to be given in bullet points. Research letters can have one table or figure, and 6 references. It could be usually authored by not more than five authors.
Letter to the editor
These should be short and decisive observations on articles published in the immediate previous issue of the BSMMU Journal. The letter could have up to 400 words and 5 references. Abstracts within 80 words without any structure needs to be submitted. Abstracts will not be published but are required for DOI purposes. Letters are not peer-reviewed. All accepted letters are edited, and proofs will be sent out to authors before publication. It could be usually authored by not more than three authors.
Perspectives
These are peer-reviewed views, hypotheses or discussions with a clear message surrounding an issue of public health interest. Perspectives could have up to 600 words and 10 references. Abstracts within 80 words without any structure needs to be submitted. Abstracts will not be published but are required for DOI purposes. Up to three highlights needs to be given in bullet points. Perspectives can have up to two tables or figures. It could be usually authored by not more than five authors. Other Editorials and Guest Editorial are solicited by the editorial board.
Viewpoints
The section includes a reaction and issue relating to JNMA, be it a comment relating a recent article, an elaboration of an important discovery, or simply a thought-provoking commentary of fewer than 1000 words without an abstract.
References
BJMR uses the Vancuvar Referencing system. It should be numbered consecutively in the order in which they are first mentioned in the text (not in alphabetic order). Identify references in text, tables, and legends by Arabic numerals in superscript with after the punctuation marks. References cited only in tables or figure legends should be numbered in accordance with the sequence established by the first identification in the text of the particular table or figure.
The titles of journals should be abbreviated according to the style available in MEDLINE/PubMed database. Use complete name of the journal for non-indexed journals. Avoid using abstracts as references. Information from manuscripts submitted but not accepted should be cited in the text as "unpublished observations" with written permission from the source. Avoid citing a "personal communication" unless it provides essential information not available from a public source, in which case the name of the person and date of communication should be cited in parentheses in the text. Include the last names and initials of the authors, title of article, name of publications, year published, volume number, and inclusive pages.
Provide a website link to all references for PubMed, PMCID, DOI, Full Text (provide all or as much as available).
Tables
Tables should be self-explanatory and should not duplicate textual material.
Tables with more than 10 columns and 25 rows are not acceptable.
Number tables, in Arabic numerals, consecutively in the order of their first citation in the text and supply a brief title for each.
Do not use internal horizontal or vertical lines.
Give each column a short or an abbreviated heading.
Place explanatory matter in footnotes, not in the heading.
Explain in footnotes all non-standard abbreviations that are used in each table.
For footnotes use the following symbols, in this sequence: *, †, ‡, §, ‖, ¶, **, ††
Tables with their legends should be provided at the end of the text after the references. The tables along with their number should be cited at the relevant place in the text.
Identify statistical measures of variations, such as standard deviation and standard error of the mean.
Be sure that each table is cited in the text.
If you use data from another published or unpublished source, obtain permission and acknowledge that source fully. Submit such tables for consideration with the paper so that they will be available to the peer reviewers
Figures (Illustrations)
BJMR accepts both colour and black & white illustrations.
Figures or illustration will be placed on the manuscript, and upload the seperate image or illustrations files in JPEG format. The file size should be within 2048 kb (2 mb) in size while uploading.
Illustrations should have sizes that match either one-column or two-column layout: 8–8.5 cm and 17–17.5 cm, respectively.
All illustrations should have a resolution of at least 300 dpi.
Figures should be numbered consecutively according to the order in which they have been first cited in the text.
Labels, numbers, and symbols should be clear and of uniform size. The lettering for figures should be large enough to be legible after reduction to fit the width of a printed column.
Do not add shading or grids to the background of graphs.
Symbols, arrows, or letters used in photomicrographs should contrast with the background and should be marked neatly with transfer type or by tissue overlay and not by pen.
Titles and detailed explanations belong in the legends for illustrations not on the illustrations themselves.
When graphs, scatter-grams or histograms are submitted the numerical data on which they are based should also be supplied.
The photographs and figures should be trimmed to remove all the unwanted areas.
If photographs of individuals are used, their pictures must be accompanied by written permission to use the photograph.
If a figure has been published elsewhere, acknowledge the original source and submit written permission from the copyright holder to reproduce the material. A credit line should appear in the legend for such figures.
Legends for illustrations: Type or print out legends (maximum 40 words, excluding the credit line) for illustrations using double spacing, with Arabic numerals corresponding to the illustrations. When symbols, arrows, numbers, or letters are used to identify parts of the illustrations, identify and explain each one in the legend. Explain the internal scale (magnification) and identify the method of staining in photomicrographs.
The Journal reserves the right to crop, rotate, reduce, or enlarge the photographs to an acceptable size.
Anknowledgement
For contributions made by individuals who are not authors, one or more statements should be included to address the following aspects: 1) Contributions that warrant acknowledgment but do not meet the criteria for authorship, such as general support from a departmental chair; 2) Recognition of technical assistance provided; and 3) Acknowledgment of financial and material support, with a clear indication of the nature of the support received. The specific details of these non-author contributions can be cited individually or collectively, along with a clear specification of their precise involvement.
The corresponding author holds the responsibility of obtaining written permission from all individuals acknowledged in the manuscript. This ensures that they have granted consent to be acknowledged for their respective contributions.
Author contribution
Authorship credit should be based only on substantial contributions to each of components mentioned below:
a) concept and design of the study
b) acquisition, analysis and interpretation of data
c) manuscript drafting and revising it critically
d) approval of the final version of the manuscript, and
e) guarantor accuracy and integrity of the work
Participating solely in obtaining funding or gathering data does not meet the criteria for authorship. Merely overseeing the research group in a general capacity is insufficient for being listed as an author. Each contributor should have actively and significantly engaged in the work to the extent that they can publicly assume responsibility for relevant portions of the manuscript's content. The sequence of contributors' names should reflect their relative contributions to both the study and the writing of the manuscript. Once the manuscript is submitted, the order of authorship cannot be altered without obtaining written consent from all the contributors involved.
Authors' contributions will be printed along with the article. One or more author should take responsibility for the integrity of the work as a whole from inception to published article. Contribution in “c” and “d” for all authors is obligatory, while the other credits are case based. The ‘author contributions’ section is not required when there is only one.
Funding
Manuscripts must provide comprehensive information about the funding agency or sponsors, including the grant number, as well as a clear description of the role played by the funders in the study. In cases where the funders had no involvement or the study received no funding, a statement explicitly stating this should be included in the manuscript.
Conflict of interest
For all types of manuscripts submitted to the journal, including articles, original research reports, editorials, comments, reviews, book reviews, and letters, it is mandatory to include a statement disclosing any potential conflicts of interest. Alternatively, authors should declare if they have no conflicts of interest to disclose. All authors of an article must disclose any and all conflicts of interest they may have with publication of the manuscript or an institution or product that is mentioned in the manuscript and/or is important to the outcome of the study presented. Authors should also disclose conflict of interest with products that compete with those mentioned in their manuscript.
Ethical approval
For studies involving human participants, it is essential to specify whether the procedures adhered to the ethical standards of the responsible committee on human experimentation (institutional or regional) and the Helsinki Declaration of 1975, as revised in 2000 (accessible at https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/). In prospective studies with human participants, authors are expected to mention approval obtained from the appropriate Ethics Committee or Review Board (regional, national, institutional, or independent), along with obtaining informed consent from adult research participants and assent from children aged over 7 years participating in the trial. The age at which assent is required may vary based on regional and/or national guidelines. Participants' confidentiality must be maintained, avoiding the use of names, initials, or hospital numbers, especially in illustrative material.
In the case of experiments involving animals, authors should indicate whether they followed the institution's or a national research council's guidelines on the care and use of laboratory animals, or any applicable national laws. On demand, authors should provide evidence of approval from a local Ethics Committee for both human and animal studies. Animal experimental procedures should be conducted with humane practices, and authors must clearly state the details of anaesthetics and analgesics used. The ethical standards of experiments must align with the guidelines provided by the CPCSEA (for studies involving experimental animals) and the World Medical Association Declaration of Helsinki on Ethical Principles for Medical Research Involving Humans (for studies involving human participants). The journal will not consider any paper that is deemed ethically unacceptable. A section titled 'Ethical Approval' should be included in all research articles, providing a statement on ethics committee permission and adherence to ethical practices.
ORCiD ID
The ORCiD IDs of all authors needs to be given.
List of acronyms
Include a list of acronyms along with its description used in the manuscript. This will not be published but required for the review process.
Data availabilty statement
All manuscripts should include a statement about where data supporting the results reported in a published article can be found.
Protection of patients' rights to privacy
Identifying information should not be published in written descriptions, photographs, sonograms, CT scans, etc., and pedigrees unless the information is essential for scientific purposes and the patient (or parent or guardian, wherever applicable) gives informed consent for publication. Authors should remove patients' names from figures even if they have obtained informed consent from the patients in order to protect patient privacy. The journal abides by ICMJE guidelines:
Authors, not the journals nor the publisher, need to obtain the patient consent form before the publication and have the form properly archived. The consent forms are not to be uploaded with the cover letter or sent through email to editorial or publisher offices.
If the manuscript contains patient images that preclude anonymity, or a description that has obvious indication to the identity of the patient, a statement about obtaining informed patient consent should be indicated in the manuscript. - In order to protect the patient's identity, the recognizable facial features not related to the study should be digitally blurred
Written informed consent is the preferred method for obtaining consent. If verbal consent is obtained, the authors must ensure that the verbal consent is recorded in the medical case record of the patient and duly signed by witness.
Editorial process
When submitting a manuscript to Bangabandhu Sheikh Mujib Medical University Journal, authors must understand that it is exclusively being considered for publication by this journal at that particular moment. The manuscript should not have been previously published, concurrently submitted elsewhere, or already accepted for publication in part or in its entirety. Authors are expected to designate one of them as the main point of contact with the Journal for all matters related to the manuscript. Upon receipt, all manuscripts are promptly acknowledged.
During the initial editorial review, submitted manuscripts are assessed for their suitability for formal peer-review. Manuscripts lacking originality, having serious scientific or technical flaws, or lacking a significant message are rejected before proceeding to the formal peer-review process. Additionally, manuscripts that are unlikely to be of interest to BJMR readers may be rejected at this preliminary stage.
Manuscripts received from Editorial Board members will be screened by the Editor in Chief and sent to external peer reviewers. The editorial board members who are authors of a submitted manuscript will be excluded from publication decisions.
Manuscripts that are found suitable for publication in BJMR are sent to two or more expert reviewers. During submission, the contributor is requested to provide names of two or three qualified reviewers who have had experience in the subject of the submitted manuscript, but this is not mandatory. The reviewers should not be affiliated with the same institutes as the contributor/s. However, the selection of these reviewers is at the sole discretion of the editor. Every manuscript is also assigned to a member of the editorial team, who based on the comments from the reviewers takes a final decision on the manuscript. The comments and suggestions (acceptance/ rejection/ amendments in manuscript) received from reviewers are conveyed to the corresponding author. If required, the author is requested to provide a point by point response to reviewers' comments and submit a revised version of the manuscript. This process is repeated till reviewers and editors are satisfied with the manuscript. Manuscripts accepted for publication are copy edited for grammar, punctuation, print style, and format. Page proofs are sent to the corresponding author. The corresponding author is expected to return the corrected proofs within three days. It may not be possible to incorporate corrections received after that period. The whole process of submission of the manuscript to final decision and sending and receiving proofs is completed online. To achieve faster and greater dissemination of knowledge and information, the journal publishes articles online as 'Ahead of Print'.
Anti plagiarism policy
Plagiarism encompasses the act of presenting one's own work, in part or whole, without proper citation or misrepresenting others' ideas, words, and creative expressions as their own. The journal does not accept previously published material and will not consider for publication manuscripts that are sent simultaneously to other journals, nor redundant or duplicate publications, that is, articles that substantially overlap another already published, printed, or electronic media.
BJMR strictly adheres to an anti-plagiarism policy. Upon submission, all manuscripts undergo plagiarism checks using commercially available software. If plagiarism is detected during the peer review process, the manuscript may be rejected. If plagiarism is detected after publication, we may issue a correction or retract the paper, as appropriate. We reserve the right to inform authors' institutions about plagiarism detected either before or after publication. The overall similarity rate of a manuscript should not exceed 15 percent.
In the event of plagiarism being detected after publication, BJMR will initiate an investigation. If plagiarism is confirmed, the authors' institution and funding bodies will be informed, and the plagiarized article will be retracted. To report cases of plagiarism, individuals may contact the journal office through email.
Copy of any permission
Authors/contributors are accountable for securing permissions to reproduce any copyrighted material. Alongside the manuscript submission, authors must provide a copy of the obtained permission. Additionally, they should include copies of all published articles or other manuscripts related to the submitted work, whether in preparation or submitted elsewhere.


